The Ovarian Tumor Dataset
Introduction
The OvaTUS dataset was collected by the research team of the Signal, Information, and Multimedia Content Processing Laboratory (SigM Lab) in collaboration with the National Hospital of Obstetrics and Gynecology (NHOG) in Hanoi, Vietnam, as part of under grant number KC-4.0-45/19-25 "Research and development of a computer-aided support system for ovarian cancer diagnosis using ultrasound images". This dataset comprises ultrasound images from women who visited the hospital for ovarian tumor assessment and consented to participate in the study. To ensure the effectiveness of data collection and analysis in research and pathological diagnosis, particularly in the management and evaluation of ovarian tumors via ultrasound, the documentation of essential data fields is crucial. These data fields not only facilitate the characterization and severity assessment of tumors but also support accurate and timely treatment decision-making. Detailed information regarding ovarian tumor characteristics is vital for diagnosis, severity assessment, and treatment planning.
Detail of the OvaTUS dataset
A. Data collection
We introduce OvaTUS-V1, the first official release of our ovarian tumor ultrasound image dataset. This dataset is developed following a standardized pipeline for data collection, storage, and annotation, ensuring consistency and reliability across all samples. All procedures strictly comply with ethical guidelines for biomedical research involving human subjects. OvaTUS-V1 is designed to support ongoing and future research in medical image analysis and will continue to be expanded with additional cases and annotations, while preserving the integrity of the data and adherence to medical ethics throughout the dataset’s evolution. The process of collecting:
- Step 1: We recruit volunteers aged 20–70, inform them about the study, and obtain their consent to use their medical results.
- Step 2: Patients undergo preoperative CA125 and HE4 biomarker testing, along with ultrasound imaging. Only those diagnosed with organic ovarian tumors and indicated for surgery, with tumor evaluation based on the IOTA model, are included.
- Step 3: Patients with functional ovarian cysts, pregnancy, end-stage renal failure, or a history of organ transplantation are excluded.
- Step 4: Surgery and postoperative histopathological analysis confirm the tumor's final diagnosis, and retain samples from confirmed benign or malignant cases, excluding those with discrepant pre- and postoperative diagnoses. Surgery is performed, and postoperative histopathological analysis is conducted to confirm the final diagnosis of the tumor. We retain samples from patients diagnosed with benign or malignant tumors. Patients initially diagnosed with ovarian cancer preoperatively but later confirmed with a different pathology postoperatively are excluded.
- Step 5: We apply techniques to remove markers, and patients' personal data, as well as to control the quality of the images before passing them for annotation. The final dataset is stored on a cloud server.
B. Collection and storage process
The dataset utilized in this study was collected at the National Hospital of Obstetrics and Gynecology (NHOG) in Hanoi, Vietnam, a leading institution specializing in maternal and reproductive healthcare. During the clinical workflow, particularly in the second step of the ultrasound imaging process, clinicians capture and store diagnostic images on a local computer system directly connected to the ultrasound machines. These images are saved in widely used medical image formats such as DICOM, PNG, and JPEG, ensuring compatibility for both clinical use and subsequent data analysis. The majority of ultrasound images included in our dataset were acquired using two commonly deployed diagnostic imaging systems in Vietnam: the Samsung Medison W80i and the GE Voluson S6. These devices are well-regarded for their reliability and imaging precision in obstetric and gynecological applications. Specifically, the Samsung W80i offers high-resolution 2D imaging along with advanced Doppler functionalities, while the Voluson S6 supports comprehensive 2D, 3D, and even 4D imaging capabilities, which are crucial for detailed visualization of ovarian structures. The combination of these technologies allows for accurate characterization of ovarian tumors in terms of size, shape, and internal structure, thereby enhancing both diagnostic accuracy and the quality of data used for computational analysis in this research.
C. Data pre-processing and annotation
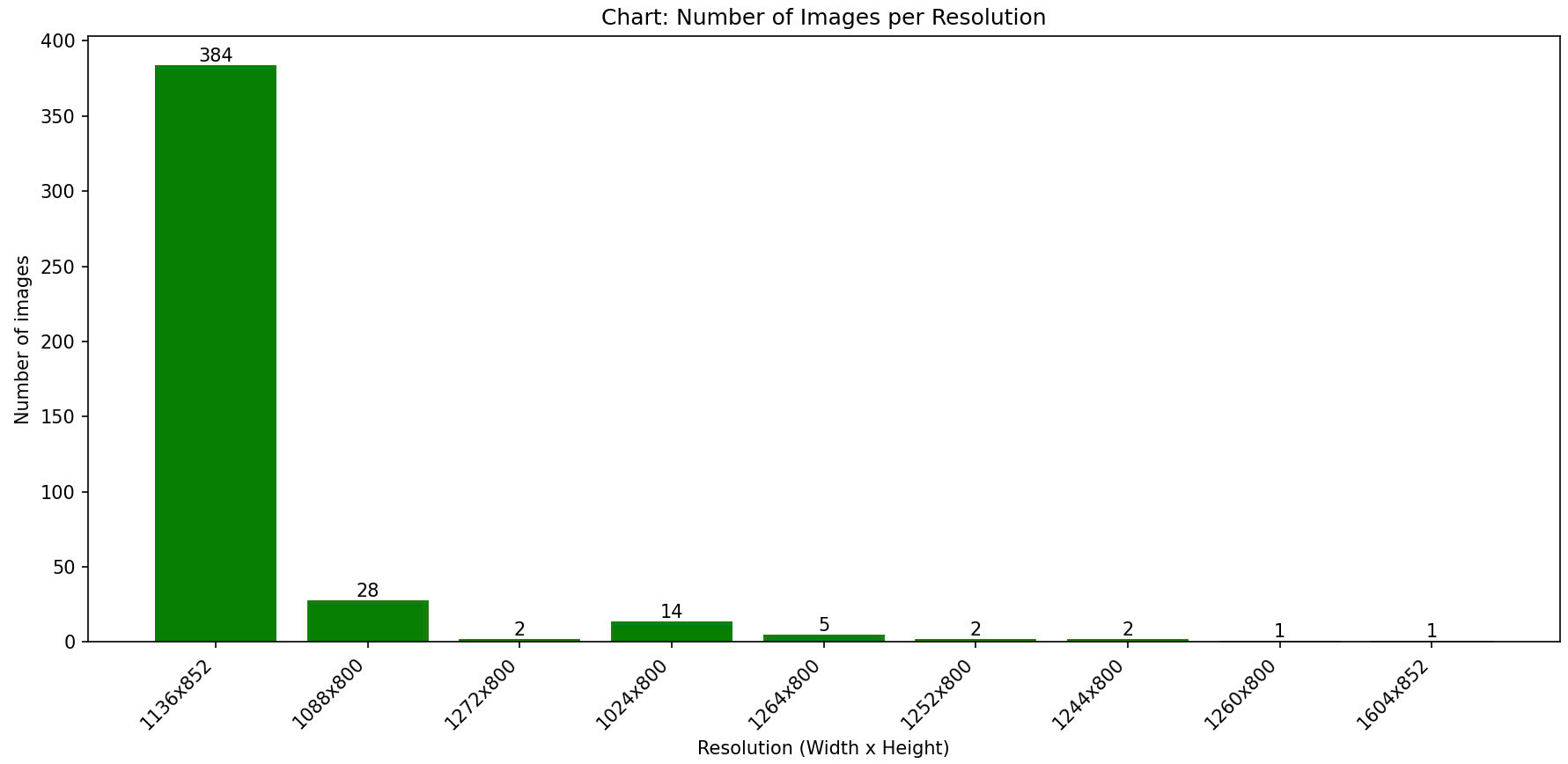
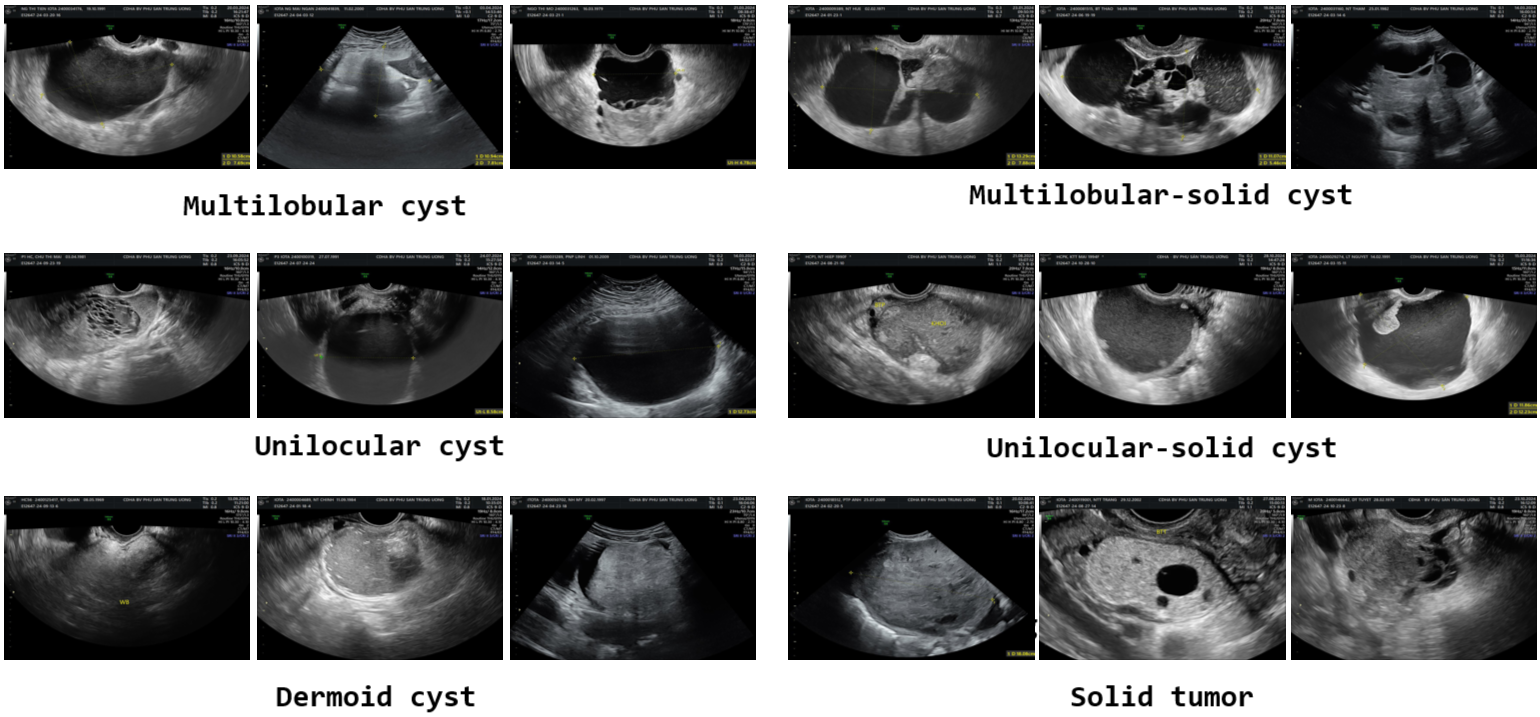
The captured images undergo pre-processing before annotation. This pre-processing step includes removing personal data and eliminating markers added by doctors during ultrasound imaging. We utilize available IOPaint tool for inpainting the images, ensuring a clean and standardized dataset for annotation. Then the doctors use LabelMe tool for tumor region annotation. LabelMe is a free and convenient tool developed by MIT CSCI labs presents the LabelMe interface as used by doctors, where the red line delineates the tumor boundary. During the annotation process, doctors followed Vietnamese standards and consulted the IOTA standards for ovarian tumor classification which includes 6 types of tumors: Solid tumor, Multilocular cyst, Unilocular cyst, Dermoid cyst, Multilocular-solid cyst and Unilocular-solid cyst. Number of samples per class in OVATUS-V1 dataset is summarized in table:
| No. | Category | Description | Number of Images |
|---|---|---|---|
| 1 | Solid tumor | A tumor with a cystic structure with only one lobe, without septa, solid tissue, or buds. | 65 |
| 2 | Multilobular cyst | A cyst with at least one septation and no solid components or papillary projections. | 137 |
| 3 | Unilocular cyst | A tumor with a cystic structure with only one lobe, but with the presence of a solid part or at least one bud. | 76 |
| 4 | Dermoid cyst | Cyst with a solid appearance and thin walls, typically unilocular and exhibiting a ground-glass pattern. | 100 |
| 5 | Multilobular-solid cyst | A single-locule ovarian cyst that contains a solid component or at least one papillary projection within its structure. | 36 |
| 6 | Unilocular-solid cyst | A cyst with a single locule and a solid component or at least one papillary projection. | 25 |
D. Ethical considerations
Researchers conducting biomedical studies, particularly those involving human participants, are ethically and legally obligated to safeguard the rights, dignity, and privacy of all individuals contributing to the research. In alignment with these responsibilities, our study was designed and implemented with strict adherence to international and national ethical guidelines. A foundational principle guiding our research process is the respect for patient autonomy and confidentiality. We recognize that patients have an inalienable right to privacy and to be protected from unauthorized disclosure of any information related to their personal identity or medical condition. Accordingly, comprehensive efforts were undertaken to ensure that all data collected from patients at the National Hospital of Obstetrics and Gynecology (NHOG) in Hanoi, Vietnam, were handled with the highest standards of data protection and ethical responsibility.
Before the commencement of data collection, the objectives, scope, and intended use of the study were transparently communicated to both the hospital administration and relevant clinical departments. This communication included a full explanation of how the data would be used, the anonymization process, and the measures in place to prevent any misuse or accidental disclosure of sensitive information. Both institutional and patient-level consents were obtained where applicable, following established ethical protocols.
The study was officially approved by the Ethics Committee on Biomedical Research of the National Hospital of Obstetrics and Gynecology, in accordance with Decision No. 1166/CN-PSTW, dated July 28, 2023. This decision provides formal recognition that the research meets the ethical standards required under Vietnamese biomedical research regulations. Moreover, the ethical framework of this study is firmly grounded in the principles of the Declaration of Helsinki, adopted by the World Medical Association (WMA). The Helsinki Declaration serves as a globally recognized ethical cornerstone for all research involving human subjects. It establishes clear guidance on issues such as informed consent, beneficence, non-maleficence, and the necessity of maintaining confidentiality throughout and beyond the duration of the research project. In Vietnam, as in many other countries, the Declaration of Helsinki is used as a foundational document to guide Institutional Review Boards (IRBs) and Ethics Committees (ECs) in the assessment of research proposals involving human participants. To uphold the confidentiality and integrity of patient data, the research team employed a series of stringent data anonymization and protection protocols. All personally identifiable information (PII), such as patient names, identification numbers, dates of birth, and medical record numbers, was either permanently removed or masked using appropriate truncation and encryption techniques prior to data processing. This anonymization process ensures that no individual patient can be re-identified based on the information retained in the research dataset. The ultrasound images, which form the core of this study, were curated to exclude any visual identifiers or embedded metadata that could reveal the patient's identity or clinical background. Furthermore, access to the dataset was strictly controlled and limited to authorized members of the research team. All data were stored in secure servers with multi-layered protection mechanisms, including password-protected systems, encrypted storage drives, and institutional firewall policies, to mitigate the risk of data breaches or unauthorized access. The research team underwent training on ethical data handling and privacy protection to ensure consistent adherence to best practices in human subjects research. In terms of data usage, the study was designed solely for academic and scientific purposes, with a specific focus on advancing diagnostic techniques in ovarian tumor segmentation using ultrasound imaging. No commercial exploitation or distribution of the patient data is permitted under the terms approved by the Ethics Committee. Additionally, the research findings are presented in aggregate form only, with no reference to individual patients or cases, thereby preserving the anonymity and dignity of all study participants. By implementing these safeguards, the research team not only complies with national and international ethical regulations but also reinforces a culture of trust between medical researchers and the patient community. Trust is fundamental in clinical research; it ensures continued collaboration between medical institutions and researchers, and it encourages patients to contribute to scientific progress by sharing their data under safe and respectful conditions. In conclusion, this study exemplifies a rigorous approach to ethical biomedical research by prioritizing the protection of personal data and ensuring transparency throughout the research process. From the initial design phase to data acquisition, storage, and analysis, all steps were conducted in accordance with the ethical principles set forth by the Declaration of Helsinki and the directives issued by the Ethics Committee of NHOG. These efforts collectively guarantee that the dignity, rights, and welfare of the patients involved remain fully protected throughout the duration of the study and in any future dissemination of its results.E. Data analysis
In all datasets, the annotated tumor regions exhibit high variability in size, shape, and texture, reflecting the diverse nature of ovarian tumors. Some segmented regions are not homogeneous, as they contain a mixture of hypoechoic (dark) and hyperechoic (bright) areas, creating a complex internal structure. Additionally, the manually drawn boundaries using annotation tools are not always smooth or perfectly aligned with the actual tumor contours, especially for small-sized tumors where precise delineation is more challenging. In cases of heterogeneous or multilobulated tumors, the boundaries are often difficult to distinguish from surrounding tissues, leading to potential over-segmentation or under-segmentation. These challenges highlight the intricacies of ovarian tumor segmentation, emphasizing the need for advanced deep-learning models and boundary-aware loss functions to enhance accuracy and robustness. In the following, we analyze quantitatively the datasets in terms of distribution in number of samples per class as well as in the size of tumor regions.
F. Distribution of samples per class
In the OVATUS-V1 dataset, the distribution of samples across categories is noticeably imbalanced. The OvaTUS-V1 dataset consists of six different tumor classes with a total of 439 images. The distribution of samples among the classes is highly imbalanced, with the "Multilocular cyst" having the highest number of images (137), whereas the "Unilocular-solid cyst" class has only 25 samples. The imbalance in class representation in OvaTUS-V1 may impact segmentation performance, requiring techniques like data augmentation or weighted loss functions to mitigate bias.
G. Distribution of tumor sizes
OvaTUS-V1 has quite a balanced distribution, with the highest frequency in the 20-30% size range, suggesting that medium-sized tumors are the most common in this dataset. These differences suggest that each dataset may have been collected under different clinical conditions, possibly reflecting variations in patient demographics, imaging protocols, or tumor detection criteria.